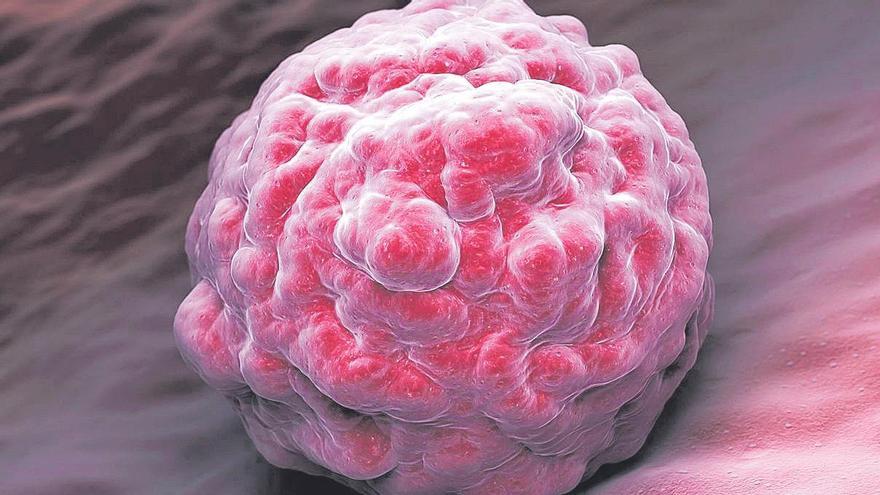
Until now, the human embryo has been obtained from eggs and sperm, either in utero or in the laboratory. Now, you can take cells from an adult and treat them to a clump that is difficult to distinguish from a fetus. Artificial embryos hold promise for studying what goes wrong during pregnancy and the origin of serious malformations. But it lives in a legal vacuum: It’s not clear whether the no-grow rule for more than 14 days, which applies to experimental embryos made from eggs and sperm, exists. Can an unscrupulous scientist implant an artificial embryo into the uterus?
And there’s more. Creating an artificial womb seems a relatively easier challenge than creating an artificial embryo. Will there come a day when these embryos can be carried outside the uterus? In the face of these questions, many scientists dislike the term artificial embryos and favor referring to stem cell-based embryo models. However, others believe that the transition from model to replica is only a matter of time. Defining the line that separates the two is not easy.
Let’s start from the beginning. The earthquake broke out in June, when biologist Magdalena Ernika Goetz announced at a conference that she had obtained artificial embryos in her laboratory at the University of Cambridge (UK). Shortly thereafter, in September, Jacob Hanna, a researcher at the Weizman Institute in Israel, published that he had achieved the same thing in another way. While Żernicka-Goetz genetically modified the stem cells, Hanna did so without genetic modification. In the following weeks, other laboratories announced that they were also working on the same thing. “We suddenly realized how many people were searching for this in the world,” says Sergio Pérez Asebron, a biologist at Heidelberg University.
Different systems
Żernicka takes pluripotent stem cells and genetically modifies them to organize themselves like embryos. Hanna doesn’t even edit genes, he mixes several types of cells in a given culture and applies a protocol that makes them structured like embryos. “Cells have genetic information to spontaneously organize themselves into structures similar to human embryos,” says Luis Montolio, a researcher at the National Center for Biotechnology.
“It’s a technique that doesn’t depend on fully understanding a living system, but on directing it to express its potential,” explains Ben Harlbut, a bioethics researcher at Arizona State University. “It’s more like gardening than engineering.”
The scientific motivation for developing an artificial embryo is to unveil a key moment in embryonic development: gastrulation, the stage in which three layers form that give rise to organs.
This stage can be observed in fish and amphibians because their gills are transparent. In mammals, it passes under the lining of the uterus and is hidden from the eyes of scientists. Artificial embryos allow us to see what happens in a laboratory dish.
“With this, in vitro fertilization can be improved and congenital diseases, such as spinal deformities, can be studied,” notes Alfonso Martinez Arias, a biologist at UPF. “Embryo models can also be used as reactors, generating all types of tissues,” says Asibron. For example, healthy eggs can be produced for women who do not have them.
Alternative system
All of this could be achieved through a less controversial system, as Martínez Arias works, among other things, on models of non-integrated embryos, artifacts that can generate a variety of tissues, which do not exactly mimic an embryo and cannot be implanted.
These models also pose ethical challenges. For example, they can be used to produce many eggs and selected for eugenics. However, it is the integrated models (such as the Hanna and Zdot;ernicka models) that generate the most controversy. Imaginable applications include producing clones or carrying an artificial fetus outside the womb. The main obstacle is technical. “The efficiency of the procedure is very poor,” says Montolio. “Only one embryo is obtained in dozens of attempts.” Martinez-Arias explained that transplantation experiments in mice resulted in “severely damaged animals.” Attempts in macaques were unsuccessful.
“What is being done is greatly exaggerated,” says the scientist who advocates that non-integrated models are more reliable and reproducible. “It is dangerous and could harm research.”
However, the technical hurdles could soon fall. “Some cell models look a lot like embryos, and they will look more and more like them,” warns Pérez-Acebron. “The specialist will spot the differences, but the truth is that they are very similar,” Montoliu agrees. Are they embryos like others or not? Many scientists agree on one criterion: embryos should be considered if, once implanted, they can give rise to an embryo. If not, they are not. Since their viability is now in doubt, many believe they should be called embryonic models.
“It’s a way of using language to avoid having to face certain problems,” answers Inmaculada de Mello-Martin, a bioethicist at the National Center for Oncology Research. If the criterion is implantation, and this is legitimate, then the criterion does not apply according to Harlbut.
“Even though they are not embryos, their resemblance can generate disapproval in the public,” Asibron says. “It may feel like we are creating humans at the implantation stage.” Martinez Arias believes that complex structures such as an artificial embryo should not be created if the same can be achieved using simpler models. “The question is not whether they are embryos or not, but what the limits are and who will set them. Just because scientists created them does not mean they should be decided by scientists alone. There are questions about human dignity and our own dignity.” “Responsibility towards it. These are questions for the whole society,” concludes Hallerbot.N

“Freelance social media evangelist. Organizer. Certified student. Music maven.”